Investigational New Drugs: FDA Has Taken Steps to Improve the Expanded Access Program but Should Further Clarify How Adverse Events Data Are Used | U.S. GAO

Simplifying Paperwork and Increasing Patient Access to Oncology Compassionate Use Therapeutics - Medical Documentation Software, Clinical Documentation Improvement, CDI Clinical Documentation Improvement, CDI Program, Insight

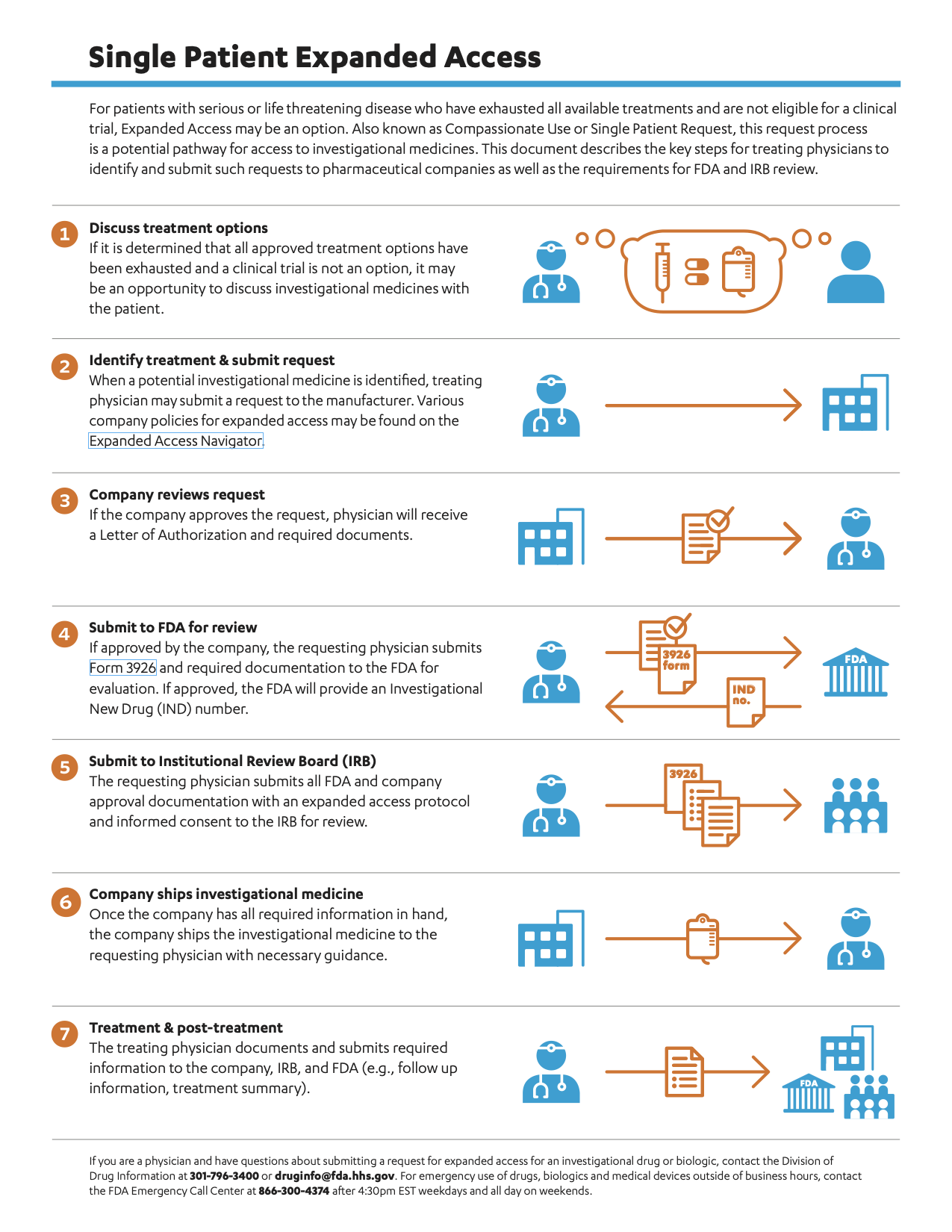
U.S. FDA on Twitter: "Have you heard of “Expanded Access?” It's a potential pathway for patients with immediately life-threatening conditions to gain access to an investigational medial product. Here's how the process

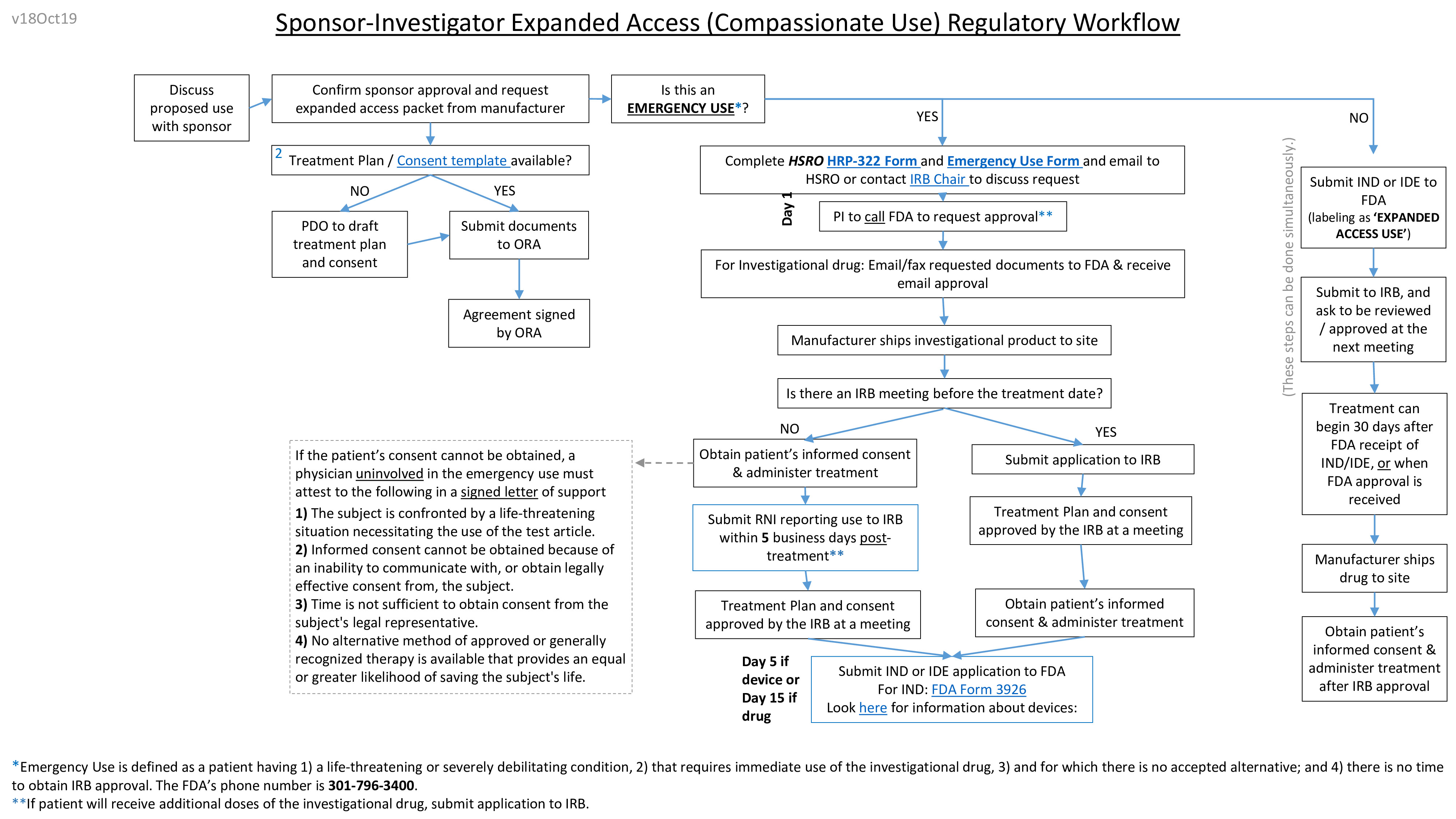
Do Your Patients Need Access to Investigational Drugs, Biologics, or Medical Devices Through FDA's Expanded Access Pathway? — MICHR

Expanding Patient Access to Investigational Drugs: Single Patient Investigational New Drug and the “Right to Try” - ScienceDirect